This is part of our Ultimate Guide to Private Label Probiotics, a complete overview of the benefits of probiotic supplementation for consumers, some of the different strains and their specific uses, the process of responsibly manufacturing a probiotic product, and what to look for when choosing a probiotic manufacturer.
Creating market-ready private label probiotics requires dietary supplement manufacturers to truly understand the needs of the customer, what trends are relevant, and which new ingredients might provide the biggest benefits.
Once the target customer has been identified and the product positioning has been established, the manufacturer must use their experience and expertise to select the right strains for the product in order to deliver the desired benefits.
They must also understand the individual needs of those strains and manage the environmental conditions the probiotics will encounter as they are changed from the raw material into finished products.
Meeting Market Needs

The probiotics market is expected to reach $74.69 Billion (USD) by 2025. As consumers become more educated about the benefits of specific probiotic strains, they are seeking out products that target specific health conditions. The use of probiotics as an alternative to pharmaceuticals to target those conditions is contributing to this market growth, and also to the ongoing research being done with different strains.
Probiotic supplement manufacturers are closely following this research in order to have new products ready to target these specific health benefits as soon as clinical support is available. They start studying and working with the strains early on, so they can create and refine the ideal formulation to deliver a safe, effective, and viable finished product. They also understand what can be said on the label of the product, clearly communicating the benefit of the private label probiotic without using any risky language or making any unsubstantiated claims.
Sourcing the Right Probiotic Strains

While there are many different probiotic species and strains available today, not all of them lend themselves to being manufactured into a supplement form. Some of them cannot be combined with other strains, as they will either kill or be killed by them. Some of them require such a high amount to offer any real health benefits that they either become cost-prohibitive to include, or it would require too many capsules to be taken. Some of them simply can’t survive the processing. Thankfully, there are experienced manufacturers who have already done all of this research and created proven, market-ready formulas available as private label probiotics.
The majority of organisms currently included in dietary supplement probiotic products are Lactobacilli and Bifidobacteria. These probiotic strains have been widely studied for their health benefits and are a popular choice for use in dietary supplements. Also included for their hardiness are the Bacillus species, which have the ability to protect themselves when conditions are not favorable, such as potentially harmful (or deadly) stomach acids and enzymes.
Some manufacturers will only work with trusted raw material suppliers that they have a proven record of success with (rather than using several different suppliers). Often, they choose to work with patented probiotic strains that have supportive research and studies. Each type and strain of probiotic can offer specific health benefits for conditions such as digestion, immunity, allergies, or heart health, and can be included in the formulation with the purpose of entering the GI tract at different points to ensure they remain intact. These strains should have research supporting their health benefits, and data showing their compatibility (they won’t kill each other if combined) and survivability.
An experienced manufacturer will be well-researched on the best probiotic raw material suppliers and the most beneficial combinations of strains for targeting different conditions and will have formulated several private label probiotic supplements accordingly. They will be able to provide the rationale for the formulation and information on the benefits of the different strains of probiotics being included. Their R&D team has spent the time making sure that the product will remain stable enough to deliver the CFU counts on the label through the product’s expiration date.
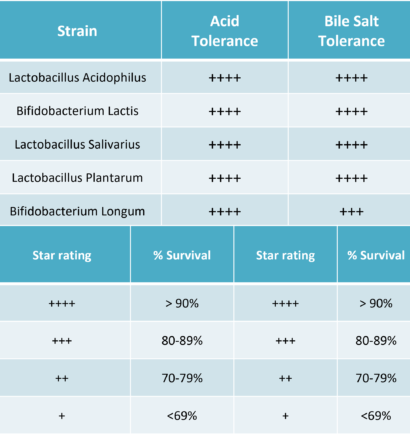
Raw Material Selection & Stability
There are several key criteria a manufacturer may use when selecting probiotics strains:
- Gastric Survivability
- Transit and Cell Adhesion
- Antibiotic Resistance
- Proven Efficacy
- Allergen-Free
Transport and Storage of Live Probiotic Organisms

Because they are living organisms, probiotics have unique handling needs at every stage of the manufacturing process. Environmental conditions, particularly heat, humidity, and oxygen are key considerations for an experienced manufacturer looking to create a viable probiotic supplement. Here are some of the steps along the way where problems are more likely to occur, and how the manufacturer works to prevent them.
- The probiotic raw materials make their journey to the manufacturer’s warehouse packed in dry ice, which keeps them cold, dry, and viable with minimal handling. Upon arrival, the material will be refrigerated (or frozen) and placed “on hold” for a quality assurance check. Besides checking the probiotic material itself, the delivery truck should also be inspected, not only for general cleanliness, hazardous chemicals, or pests but also for heat and humidity. It’s important to make sure that the organisms have survived their trip to the manufacturing facility.
- Once QA (quality assurance) releases the probiotics as approved, production can begin. The process of freezing and thawing probiotics too quickly can result in condensation, which can kill them. This can be a significant threat to the potency and viability of the product. Tempering is the process of gradually bringing the probiotic material from storage temperature to manufacturing temperature in a controlled way, which prevents condensation from developing.
- Proper temperature and humidity levels need to be maintained throughout all stages of production. A private label manufacturing facility working with probiotics will have equipment specifically for controlling temperature and humidity. Ideally, they will utilize a high-level air filtration system to manage the flow of air through the building, which helps to maintain the optimal conditions in every room that the probiotics need to be in. Additionally, production scheduling when working with probiotics should take all parts of the process into account, such as cleaning the production rooms and equipment, packaging the finished product, and storing the finished product until it ships out. During all of these steps, it is critical to be aware of, and manage, any temperature fluctuations.
- The environmental controls need to extend into the packaging area, as well. Humidity and temperature levels must be maintained at their ideal levels, and packaging should happen as soon as the probiotics enter that area, so the material isn’t sitting exposed for a long period of time.
- Once the probiotics are formulated, produced and packaged, they need to be stored AND transported in an environment that is optimal for their viability – usually on air conditioned or refrigerated trucks.
Summary
Obviously, there are many challenges and potential pitfalls involved in manufacturing with probiotics. Utilizing a private label probiotic that an experienced manufacturer has taken the time to research and develop is the best way to guarantee a safe and effective finished product.
Key Takeaways
- Private label supplement manufacturers need to keep up with consumer trends and scientific research to identify which private label probiotic products to create.
- It is important to select the appropriate strains for a probiotic formula in order to provide the desired health benefit and create a viable, effective finished product.
- Live probiotic organisms must be transported, stored, and packaged at optimal temperatures and humidity levels to remain viable.
- An experienced and knowledgeable manufacturer will create a safe and effective private label probiotic supplement that meets label claims, is scientifically supported, and addresses the needs of individual consumers.
➤ Learn More About Quality Control and Testing for Private Label Probiotic Supplements.

†These statements have not been evaluated by The Food and Drug Administration. This product is not intended to diagnose, treat, cure or prevent any disease.